扫一扫 添加小助手
服务热线
13818320332
扫一扫 关注我们

ISPE指南《生产记录数据完整性》给出关于生产数据的审核要求,如下:
应在文件中明确记录审核的过程
应在文件明确哪些数据应该被审核
所有通过和失败的测试(如过滤器完整性测试)或所有成功的、重复的、中止的运行或程序(如CIP)应包含在生产记录中以供审核
解释如何审核各系统的时间同步性
应该尽可能使用自动化解决方案来检查数据审核项目
关于哪些数据需要被审核,提供了示例描述不同类型审核过程需要审核的项目:
翻译:nancy
校对:无影、流浪的沙子
Detecting Data Integrity Issues
检查数据完整性问题
Data Review Requirements
数据审核要求
Define the Process for Data Review in a Procedure
在文件中规定数据审核的过程
The process for review should be described in a procedure [11] as set out in Section 4.4 of the ISPE GAMP® Guide: Records and Data Integrity [3]. Manufacturing data review is unlikely to be straight forward and the procedure may need to address:
应根据ISPE GAMP®指南:记录和数据完整性[3] 第4.4节,在文件中明确审核的过程。生产数据的审核不大可能简单直接,规程应描述:
Which data belongs to which of the many systems involved in the production process
在生产过程中涉及的各个系统中,哪些数据属于哪个系统;
The order in which data must be reviewed, for example, if it is stored across several systems and includes paper generated forms or reports
哪些数据应被审核,例如,当储存于多个系统并且包括走纸记录或报告;
How all passed and failed tests (e.g., filter integrity tests) or all successful, repeated, aborted runs or cycles (e.g., CIP) will be included with the production record for review to ensure full visibility to the reviewers
所有通过和失败的测试(如过滤器完整性测试)或所有成功的、重复的、中止的运行或程序(如CIP)将如何包含在生产记录中以供审核,以确保对审核人员完全可见;
An explanation of how to review the time synchronization across multiple systems
解释如何审核各系统的时间同步性
An explanation on the use of trend data retrieval modes so that the right averaging or plot points are selected to ensure that meaningful data is obtained (e.g., selection of data on an hourly basis rather than a daily basis will lead to a very different type of review)
使用趋势数据检索模式的解释,以便选择正确的平均值或标绘点,以确保获得有意义的数据(例如,选择每小时而不是每天的数据,将导致不同的审核类型)。
Details on how the review is approved, especially if it involves several different departments, for example production and engineering
详细说明审核如何获得批准,特别是涉及多个不同部门,比如生产和工程;
Details on how errors or omissions detected are escalated, particularly if data issues are found relating to a system managed by a supplier
关于检测到的错误或遗漏如何升级的详细信息,尤其是发现与供应商管理的系统相关的数据问题;
Product release by exception and the implications for further data review
异常产品放行和对进一步数据审核的影响;
As far as possible, automated solutions should be used to collate items for data reviews, as this mitigates the risks associated with human correlations, interpretations, or manual extraction. For new manufacturing systems, this requirement should be a priority in the design.
应该尽可能使用自动化解决方案来检查数据审核项目,因为这可以降低与人员的相关性、主观性或手动选取的相关风险。设计新的制造系统时应优先考虑该要求。
On existing systems when there is a clearly identified requirement for frequent review of certain data items not currently automated, upgrading the systems should be considered so that review items are collated automatically and presented in a manner that makes the review easy. An example is the automatic extraction of alarm data that corresponds to each batch. There will be a cost to this but the long-term benefit is likely to be significant.
在现有的系统上,当有明确的需求需要频繁地检查某些当前没有自动化的数据项时,应该考虑升级系统,以便自动地检查审核项,并以易于审核的方式呈现。例如,自动提取与每个批对应的报警数据。这将增加成本,但可能获得显著的长期效益。
Data used in the automatic control of normal day-to-day processes or equipment may be short term and not stored, and therefore cannot be subject to review. The appropriate controls should have been verified during initial validation. Visual data only displayed instantaneously on a process control screen falls into this category. An example of how this is documented and handled is shown in Appendix 1, Table 8.1.
用于日常过程或设备自动控制的数据可能是短期的,不能存储,因此不能进行审查。应该在首验证期间确认适当控制。只在过程控制屏幕上即时显示的可视数据属于这一类。附录1表8.1中显示了如何记录和处理这一过程的示例。
Types of Data to Review
需要审核的数据类型
The data for review can be grouped as follows:
需要审核的数据可分类如下:
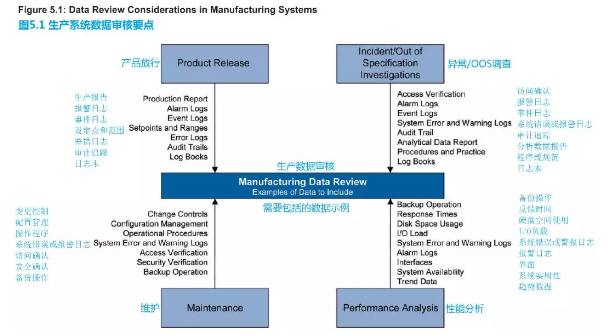
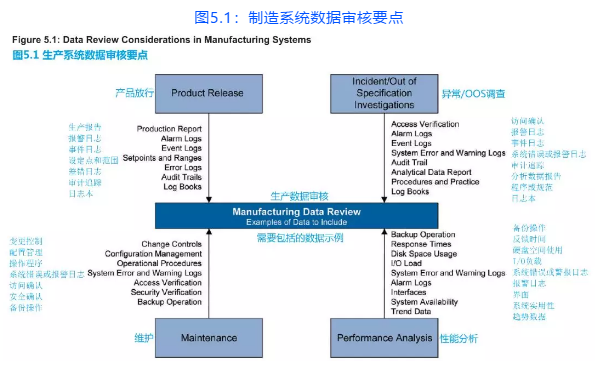
Product release data
产品放行数据
Support of incident and OOS investigation data
支持事件或OOS调查的数据
Maintenance data
维护数据
Performance analysis data
性能分析数据
Figure 5.1 gives examples of items to review. What is reviewed, at what level, and at what frequency, is determined using a risk-based approach.
图5.1提供了需要审核的项目示例。审核内容,审核级别,审核频次都应采用基于风险的方法。
Figure 5.1: Data Review Considerations in Manufacturing Systems
文章来源: GMP办公室
本网站刊载的所有内容,包括文字、图片、音频、视频、软件等,如非标注为“原创”,则相关版权归原作者所有,如原作者不愿意在本网站刊登相关内容,请及时通知本站,我们将第一时间予以删除。