扫一扫 添加小助手
服务热线
13818320332
扫一扫 关注我们
 One of the red rags to a regulatory bull is the issue of manual integration of chromatograms. If seen during an inspection, you can almost see the wheels in the inspector’s brain turn in mechanical precision as they question: are they testing into compliance? In a 2014 FDA inspection of a laboratory in Europe, one FDA 483 observation stated:
One of the red rags to a regulatory bull is the issue of manual integration of chromatograms. If seen during an inspection, you can almost see the wheels in the inspector’s brain turn in mechanical precision as they question: are they testing into compliance? In a 2014 FDA inspection of a laboratory in Europe, one FDA 483 observation stated:
色谱手动积分问题就像是斗牛中的一块红布。如果在检查期间被发现,你几乎能够看到检查官的脑中条件反射地联想到一个问题:他们的检验是否合规?在2014年FDA检查一个欧洲试验室时,一个FDA483的缺陷项如下:
No procedure exists describing how to perform manual integration.
没有关于怎样进行手动积分的规程。
A more serious non-compliance occurred in the FDA warning letter to Leiner Health Products:
在对Leiner Health Products 的FDA警告信中提到一个更加严重的不符合项:
In addition, our investigators documented many instances with extensive manipulation of data with no explanation regarding why the manipulation was conducted. This manipulation would include changing integration parameters or relabelling peaks such that previously resolved peaks would not be integrated and included in the calculation for impurities.
此外,我们调查人员记录了许多关于修改数据的案例,他们都没有解释为什么修改。这些修改包括改变积分参数或者重新标定峰以至于原来峰的分离度不能进行积分和杂质计算。
There have been many other cases that I summarized in a recent Questions of Quality column on the role of chromatography data systems (CDS) in data falsification. What regulatory guidance is there to help us? We need to consider some basics of integrating chromatographic peaks before we can look at manual integration and the regulatory guidance surrounding it. The reason for this is simple: if integration parameters are set correctly and the chromatography is acceptable then there should be no need to reintegrate manually in many cases. However, we also need to acknowledge that chromatographic systems are dynamic by their nature and separations can change during a run, so getting the right overall integration can be a balancing act.
还有一些其他情况,我近期总结了关于一个色谱数据系统(CDS)在数据造假中的作用的质量问题专栏。在这里有什么法规指南能够帮助我们?我们在关注相关积分手册和法规指南之前需要先了解一些色谱积分的基础问题。这样做的理由很简单:如果正确设定积分参数和可接受的色谱图,那么一些情况就没有必要进行手动重新积分。但是,我们需要承认在检测运行期间色谱系统的特性是动态的和分离是可变的,因此正确全面的设定积分能起到平衡的作用。
The regulators are wrong in their requirement for a procedure on manual integration. What is required instead is a standard operating procedure (SOP) for chromatographic integration, of which manual integration is an important sub-set. Although the focus of this column is manual integration please do not lose sight of the bigger picture. As such, we will not be discussing the various calibration methods that could be applied to standards to quantify analytes in samples nor will we be looking at analogue to digital conversion because the latter has been covered in an earlier Questions of Quality column . Furthermore we will not be considering the use of system suitability tests (SSTs), again because this column has already discussed them . The paper by Hill et al. on manual reintegration in bioanalysis is also a highly recommended publication to read on the subject.
监管者对手动积分规程的要求是错误的。这些要求对色谱积分的标准操作规程(SOP),而不是手动积分的要求,手动积分是另外一个重要分支。虽然专栏的焦点是手动积分,但是我们要比较全面的来看待问题。因此我们将不讨论适用于标定样品定量分析使用的各种校验方法,也不去关注类似的数据转化因为这些已经在之前的质量问题专栏中提到过了。此外我们也将不考虑系统适用性(SSTs)的使用,因为已经再其他期的专栏中讨论过了.本文强烈推荐去阅读Hill et al关于生物分析的手动重新积分的课题.
Back to Integration Basics
色谱积分基础
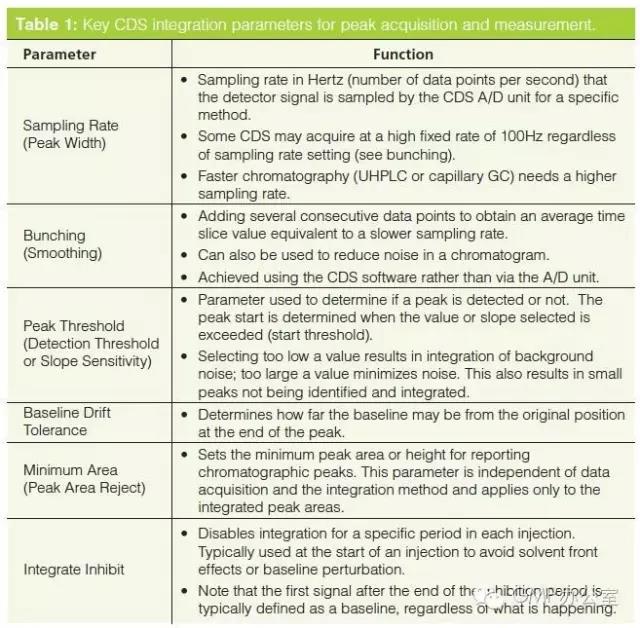
Before we can discuss how to control and manage manual integration it is important to understand the basics of chromatographic integration itself. The best book on the subject of integration is by Norman Dyson and, although the second edition was published in 1998, it is still applicable and highly recommended if you want to understand chromatographic integration. The key integration parameters are shown in Table 1 and the main ones are discussed below. Note that some CDS suppliers may call these parameters a different name but the functionality is essentially the same.
之前我们讨论如何控制和管理手动积分,这对于色谱积分本身基础的理解是十分重要的。对于积分这个课题最好的书是Norman Dyson 的著作,在1998年已经发行了第二版,它仍旧使用。如果你想了解色谱积分强烈推荐这本书。关键积分参数在表1中列出,并且一些重要的部分在下面进行了讨论。值得注意的是不同的CDS供应商对他们的参数有不同名称,但是功能的本质是相同的。
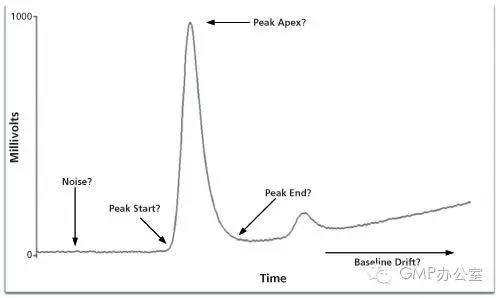
图1:峰的关键参数
对于峰值采集和测量CDS积分的关键参数
Chromatographic integration begins when the CDS samples the detector output using an analogue to digital converter (A/D). An integration method in the CDS determines the frequency of the detector data collection and analysis run time. Dependent on the CDS, either an integration method or a processing method will be automatically applied to the file of data slices to calculate the peak areas or heights. The processing method will contain the identities of the peaks of interest and their expected elution time windows. These data files and their interpretation constitute part of the raw data and primary record for the analysis. We will discuss the definition of primary record in the next Questions of Quality column in light of the two MHRA data integrity guidance documents issued this year .
当检测器用一个类似于数字转换器(A/D)的装置开始输出信号时色谱积分开始。在CDS中积分的方法确定了检测器收集频率和分析运行时间。依靠CDS,积分方法或处理方法将自动应用于峰面积或高度的计算积分。处理方法包括对目标峰的鉴别和期望在界面中的洗脱时间。这些数据文件和解释组成了分析的原始数据和最初记录。我们将讨论在下一个质量问题专栏中讨论最初记录的定义,根据MHRA发布的数据完整性指南。
The two most important parameters for integration are sampling rate and peak threshold. In combination they determine the slices for peak measurement but also suppress noise to allow better peak measurement .
采样率和超过阈值角是积分的两个最重要参数。结合他们两个决定了峰测量,但抑制噪声能够使峰得到更好的测量。
Peak width or sampling rate setting governs how often the detector output is sampled, which directly impacts the accuracy of peak area measurement. If the sampling rate is too slow it could result in the integration missing a small fast eluting peak or a valley between two peaks. Conversely, too fast a data acquisition rate can be managed by data bunching in the CDS software. Typically you should sample a peak at least 20–30 times across its width so that it can be integrated accurately; for example, conventional high performance liquid chromatography (HPLC) typically requires a 0.5–1 Hz sampling rate, faster capillary gas chromatography (GC) has a sampling rate in the range 5–20 Hz, and ultrahigh-pressure liquid chromatography (UHPLC) needs a sampling rate between 20–50 Hz. Most A/D units in CDS are rated at up to a 100 Hz sampling rate.
切线峰宽(peak width)和采样率的设定控制检测器采样的输出频率,并对峰面积测量的准确度有直接影响。如果采样率太慢,可能导致积分不到小的洗脱峰和两峰之间的低谷。相反,太快的采样频率会被CDS软件中的数据聚束所管制。一般情况下,至少20-30次采样才能准确的积分。例如,传统HPLC一般要求0.5-1HZ的采样率,快速毛细管气相GC的采样率范围5-20HZ,UHPLC的采样率范围是20-50HZ.大多数在CDS中的A/D中的采样率上限是100HZ.
Peak threshold is the setting that discriminates the start and end of a peak from baseline noise. It is based on the rate of change when the detector signal rises above a preset value in the integration method for peak start or falls in the case of peak end. This needs to be set carefully because if the baseline is noisy then noise is detected as peaks; if set too large the integration will miss small peaks because the threshold is not triggered. The same process occurs at peak end but if the threshold is low, too much of the peak tailing is counted. Conversely if the threshold is set too high the peak finishes early, which under-estimates the area of the peak.
超过阈值角(Peak threshold)设定用于辨别一个峰在基线噪音上开始和结束。它根据当检测器发出大于以上预定值的信号时发生频率的改变在积分方法中由于峰的开始或峰底部下滑的情况。他需要小心设定,如果不小心基线被检测成噪音,然后噪音被检测成峰;如果设定太大积分将可能错过小峰,因为阈值没有响应到。相同的程序也可能发生在峰底部,如果阈值设定太低,就有太多的峰尾被记录。相反如果阈值设定过高,峰会很早结束,峰面积会被低估。
Once the peak has been detected the next step is to determine when it ends. Typically this is when the signal returns to where the baseline was before the peak started but if there are several peaks eluting or the baseline is rising then the signal will not return to the baseline origin. Therefore baseline drift tolerance determines how far the baseline can drift from the original position. This is in contrast to the peak threshold, which determines how fast it can drift away (8).
一旦峰被检测到下一个步骤就是确定它何时结束。一般情况下信号会返回原来峰开始时的基线位置,但是如果有几个峰洗脱或者基线上升,信号将不能返回到原来的基线位置。因此基线漂移公差确定了基线能够漂移原来位置有多远。这是以超过阈值角为参照,它确定基线能漂移多远。
From this and alongside a more detailed discussion in Dyson’s book (8) we can develop three basic rules of chromatographic integration:
在Dyson’s book 中有详细的讨论,我们能够开发出色谱积分的三个基本原则:
Rule 1: Do NOT use default integration parameters. Ensure that each set of integration parameters is tailored to an individual analytical procedure and do not use a one-size-fits-all approach. For Beer‑Lambert’s law to hold, the samples and standards must be consistently integrated, otherwise the fundamental comparison of absorbance versus concentration cannot be performed.
原则1:不使用系统默认的积分参数。确定每个积分参数被设定为适应单独的分析程序,不能用一个放之四海而皆准的方法。对于Beer‑Lambert定律,样品和标准品必须连续积分,否则吸收度与浓度的对比不能执行。
Rule 2: The function of a CDS is not to compensate for your poor method development or separation. There is a belief in many laboratories that a CDS can be used to salvage an analytical run but this is not the case. Robust and validated methods should reduce or eliminate this issue.
原则2:CDS的功能不能弥补你方法的缺点。许多实验室中有一个信条,CDS能用于弥补分析方法的缺陷,其实事实并非如此。健全和经过验证的方法能减少或消除这一问题。
Rule 3: Understand what is happening in the CDS. Just because you get a number from a CDS does not mean you have to believe the result. Use your eyes to look and your brain to think. For example, look at the integration codes (BB or BV): are these the ones expected? Are baselines positioned where they should be and are they as expected? Are retention times and peak shapes consistent throughout the run?
原则3:理解CDS发生了什么的事情。因为从CDS中得到一些不明信息,但是你必须相信他的结果。所以只有用眼睛去看观察用脑子去思考。例如,观察积分代码(BB 或 BV):这是预期的吗?基线应该在什么位置,希望他们在什么位置?是否保留时间和峰的形状是否始终如一在运行中?
To complete this overview of CDS integration, please note what Dyson says: improving the chromatography must always take precedence over setting up the CDS (8):
读完CDS积分的概论,请注意Dyson说的话:改善色谱方法优先于优化CDS
If a chromatographer finds it necessary to tweak the parameters continuously in order to achieve consistent measurement of standard samples, it is a clear indication that more work is needed to bring the instrument and analysis under control.
如果一个色谱方法需要不断的调整来达到测量标准品的目的,这就说明,需要更多的工作来使仪器分析受控。
In essence the likelihood of a Leiner‑style non-compliance citation is looming. This is because excessive use of manual integration to compensate for poor method development or chromatographic separation increases risk to data integrity (accuracy), and increases time to generate, review, and release results.
其实Leiner‑style不合规的可能性正在逼近。这是因为过多的手动积分来弥补方法开发的缺点或色谱分离将增加数据完整性(精度)的风险,同时增加了生成、审核和结果放行的时间。
How Can Manual Integration Result in Falsification?
手动积分如何导致了造假?
Using manual integration to falsify chromatographic data can arise in a number of ways, but the two main ways are:
用手动积分来进行数据造假的情况正在增加,但是主要有两种途径:
Peak Shaving — Manually placing the baselines to reduce the peak area on integration to enhance the analyte amount in a sample (only the standards are shaved) or reduce the amount of analyte reported (by shaving the sample and not the standards). In Figure 2 the first eluting peak has had the baseline manually adjusted to reduce the total peak area.
峰值消减-手动设置基线来减少峰面积在积分中增加样品的分析数值(近标准品面积被消减)或者减少分析报告的数值(通过消减样品的峰面积但不减少标准品的面积)。在图2中第一个洗脱峰被进行了手动调整同减少总的峰面积。
Peak Enhancing — Adjusting the baselines for integration to increase the area of a peak. The enhancement of sample areas over the standards can increase the calculated amount in the sample. If reduction is required, the enhancement of the standards only is performed. Figure 2, line 3 shows how the minor or second eluting peak in the chromatogram can be enhanced.
峰面积增加-在积分过程中调整基线增加峰的面积。增加样品的峰面积多于标准品的面积,从而增加样品的计算数值。如果需要减少,仅增加标准品的峰面积。图2中,line3显示了怎样增加色谱图中的第二个洗脱峰。
Figure 2 shows the exaggerated enhancement and shaving of peaks but sometimes all that is required is a small change to bring a non‑conforming batch into compliance with a specification. Hence the need to trend analytical results as required under the new revision to EU GMP for Quality Control laboratories (12) to highlight out of expectation (OOE) and out of trend (OOT) results, as well as those that are out of specification (OOS). Results out of trend or expectation may identify the actions of an individual analyst, which may warrant closer inspection.
图2中显示夸大的增加和消减峰面积,所有这些都是让结果发生轻微改变从而使不符合变成符合标准。因此需要按照新版EU GMP对QC控制要求,对分析结果进行趋势分析,加强OOE,OOT和OOS结果的控制,OOT或OOE的结果可能有别于其他单个分析活动,但是可能需要更仔细的检查。
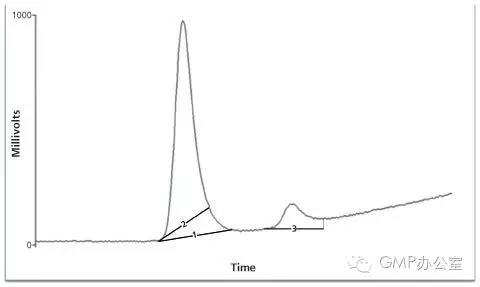
图2:峰面积增加和消减的例子
In addition, inspectors and auditors will look for a number of factors to determine if there is unauthorized manual manipulation of a peak:
另外,检查官和审计人员应该关注一些非法手动积分的相关因素:
Discovery of manual integration that is not traceable or retrievable. A chromatographer should be able to demonstrate that same results can be obtained if data files from a run are reprocessed — a red flag should be raised if this cannot be done quickly. An inspector looking to see if the CDS audit trail has been turned off temporarily to conduct falsification could also accompany this
发现一些手动积分没有追踪和不可读。色谱工作人员应该能演示获取相同的结果如果数据文件来源于一个重新处理的序列-如果不能获取,一个警告就应该出现。检查官发现CDS的审计追踪系统已经临时关闭,就可能伴随造假了。
Electronic data are not available. A focus on paper because the raw data and the electronic records have been deleted or not saved means that data cannot be reprocessed and the result confirmed. The question of falsification is raised and the laboratory is on the slippery slope to compliance hell.
电子数据不可用。因为原始数据和电子记录已经被删除或不能被重新加工而没有留存,这个结果一旦证实。造假的问题就出现了,并且实验室符合度随之下滑至谷底。
Integration parameters are different for standards and the unknown samples of the same run or between replicate injections of the same unknown sample in an analytical batch.
相同运行中标准品和未知样品设定不同的积分参数或者在批分析过程中重复进样未知批的相同样品。
Evidence that the audit trail in the system has been turned off and then on again a few minutes later: what changes have been performed that are not recorded?
在系统中关掉审计追踪几分钟后再次开启提示:改变了什么不被记录的意图?
GMP Regulations and Regulatory Guidance
GMP法规和监管指南
Although there is great interest in data integrity in laboratories working under the Good Manufacturing Practice (GMP) regulations, there is a paucity of either explicit regulation or regulatory guidance to help chromatographers.
虽然GMP管理中有许多实验室数据完整性方面的法规,但是缺乏对色谱分析的明确规定或监督指南。
The only GMP regulations applicable are the requirements for scientifically sound analytical procedures in 21 CFR 160(b) and for complete data under 21 CFR 211.194(a) . The latter topic was discussed in two earlier QOQ columns . In addition, there is data integrity audit under objective 3 in the FDA’s Compliance Policy Guide 7346.832 for pre-approval inspections . Both the European Pharmacopoeia (chapter 2.2.46)and the United States Pharmacopoeia <621> discuss criteria for good chromatography; there are no criteria for chromatographic integration or a similar discussion on data integrity. However, in the interest of scientific soundness: can you justify and defend your actions on the basis of good chromatographic science?
能使用的法规仅有科学合理的分析方法21 CFR 160(b)和完整的数据21 CFR 211.194(a) ,后者在早期的QOQ专栏中讨论过。另外,在objective 3 in the FDA’s Compliance Policy Guide 7346.832 for pre-approval inspections 中有数据完整性的审计。在the European Pharmacopoeia (chapter 2.2.46) and the United States Pharmacopoeia <621> 中说明了良好色谱分析的标准。没有相关色谱积分的标准或相似的数据完整性讨论。但是为了科学可靠的目的:您能证明和说明你的活动是基于科学的色谱分析吗?
In my view, there is a need for regulatory agencies to issue guidance on the subject of integration with a focus on manual integration for GMP. As they typically move at glacial speeds, the likelihood of this occurring before the next ice age is probably minimal. However, there is an area where regulators have been active on the subject of manual reintegration and this is in bioanalysis of samples from non-clinical and clinical studies for the registration of drugs.
我认为,这需要法规监管部门发布一个关于GMP手动积分主题的指南。但这是特别缓慢的。说不定可能会到下一个冰河世纪。但是有一个地区已经对手动积分做了行动,这就是药品注册时临床和非临床研究的样品生物分析。
Bioanalytical Regulatory Guidance for Reintegration
生物分析法规指南中的重新积分
The FDA created guidance for bioanalytical method validation following an AAPA-FDA conference in Washington in 1990, where I was involved as a co-chair, and the outcome from that meeting was a scientific paper on the subject . After a follow-up conference in 1999, the FDA issued Guidance for Industry on Bioanalytical Method Validation . In the section dealing with routine analysis of samples there is mention of sample data reintegration:
1990年FDA对生物分析方法验证建立了指南在Washington的AAPA-FDA会议中,我作为联合组长参与了这次会议,这次会议的结果见scientific paper on the subject 。后续的会议在1999年召开,FDA发布了Guidance for Industry on Bioanalytical Method Validation ,在这个部分中关于处理日常样品检验的重新积分观点如下:
An SOP or guideline for sample data reintegration should be established.
应该建立一份关于样品数据重新积分的SOP或指南。
This SOP or guideline should explain the reasons for reintegration and how the reintegration is to be performed. The rationale for reintegration should be clearly described and documented.
在SOP或指南中应该说明重新积分的原因,并说明如何进行了重新积分。重新积分的原理应该被清楚的描述和记录。
The original and the reintegration data should be reported.
初始和重新积分的数据都应该被报告
Later in the document, in the section on reports for routine studies, there is another section on Documentation for Reintegrated Data, which, in part, requires:
在后来的文档中,在日常研究的报告章节,有另外一个文档Reintegrated Data ,要求如下:
The method used for reintegration.
重新积分使用的方法
The reason for the reintegration.
重新积分的理由
The requestor of the reintegration and the manager authorizing reintegration.
重新积分的申请者和管理者授权后才能进行重新积分。
Reintegration of a clinical or preclinical sample should be performed only under a predefined SOP.
临床和临床前样品的重新积分只能在预先指定的SOP下进行操作。
In 2013, the FDA issued a draft revision of this Guidance for Industry where sample data integration was updated (please remember that the guidance is still a draft), but the key requirements are essentially unchanged. There is a rearrangement of the order listed above with the additional need to retain audit trail information from the CDS .
在2013年,FDA发布了对工业生产的指南修改起草版本,样品数据积分进行了更新(但是请注意现在这个指南依然是起草版本),但是关键的要求没有进行本质的改变。是对以上文件的重新整理文件,除了增加了审计追踪的在CDS中的保留部分。
At last we are getting somewhere! We now have some guidance albeit in the good laboratory practice (GLP) arena. But wait! There is more! Not to be outdone by their American cousins, the EMA (European Medicines Agency) produced their own guidance document on Bioanalytical Method Validation (22) in 2011. Section 5.5 is concise and devoted to the subject of integration:
最终我们将去往哪里!我们现在虽然有一些GLP (good laboratory practice)。但是还需要更多。美国和EMA发布了他们自己的指南对生物分析方法验证 in 2011。在5.5章节涉及到了积分:
Chromatogram integration and reintegration should be described in a SOP.
色谱积分和重新积分应该在SOP中进行描述
Any deviation from this SOP should be discussed in the analytical report.
所有偏离SOP的偏差都应该在分析报告中讨论
Chromatogram integration parameters and in case of re-integration, initial and the final integration data should be documented at the laboratory and should be available upon request.
色谱积分参数和重新积分的情况,初始和最终积分数据都应该在实验室进行记录并且经过申请。
Although both the FDA and EMA allow reintegration of chromatograms, it must be under controlled conditions and must be reported in the final report along with who authorized the reintegration and what the original results were together with the need for audit trail entries and justification. Personally, I prefer the European approach because the FDA guidance is over-bureaucratic. This is particularly the case as the FDA requirement for documentation of the requestor and authorization by a manager will have to be outside of a CDS because there are currently no functions available to perform this. If required by regulators this needs to be included as a function in future versions of chromatography data systems.
虽然FDA和EMA允许进行色谱的重新积分,他必须在可控的条件下进行并且必须在最终报告中体现,并且在授权的情况下进行,原始数据和重新积分的数据在审计追踪中可以查到,并且进行了说明。就个人而言,我更喜欢欧洲的指南,因为FDA太过官僚。在这件事情上是个例外,FDA要求申请和管理者授权的文件可以在CDS之外,因为目前CDS还没有这个功能可以使用。如果监管者要求,将来的色谱数据系统可能会包括这个功能。
文章来源:GMP办公室
本网站刊载的所有内容,包括文字、图片、音频、视频、软件等,如非标注为“原创”,则相关版权归原作者所有,如原作者不愿意在本网站刊登相关内容,请及时通知本站,我们将第一时间予以删除。