扫一扫 添加小助手
服务热线
13818320332
扫一扫 关注我们
 Does an OOS (OOX) examination have to be triggered if alaboratory error is ruled out and a production error has already been found anddocumented?
Does an OOS (OOX) examination have to be triggered if alaboratory error is ruled out and a production error has already been found anddocumented?
如果排除了实验室错误, 并且发现并记录了生产错误, 是否必须触发OOS(OOX) 检查?
If an error has occurred during production and has beendocumented, this should be recorded using the deviation system. Obviously,however, the product was still analysed - possibly with the expectation thatthe production error would not lead to an OOS. But if a (release) analysis isperformed and one result is an OOS, you need in any case to perform an OOSinvestigation. After all, there is also the possibility that the OOS result isdue to a laboratory error and your batch is within the specification despitethe production deviation!
如果在生产过程中发生了错误, 并已记录在案, 则应使用偏差系统进行记录。但是,怀着生产偏差不一定导致OOS结果的期望,仍对该产品进行了检验。但是, 如果执行了 (放行) 检验, 并且有一个结果是OOS,则在任何情况下都需要执行OOS调查。毕竟, 也有可能OOS结果是由于实验室错误, 而你的批次是合格的, 尽管有生产偏差!
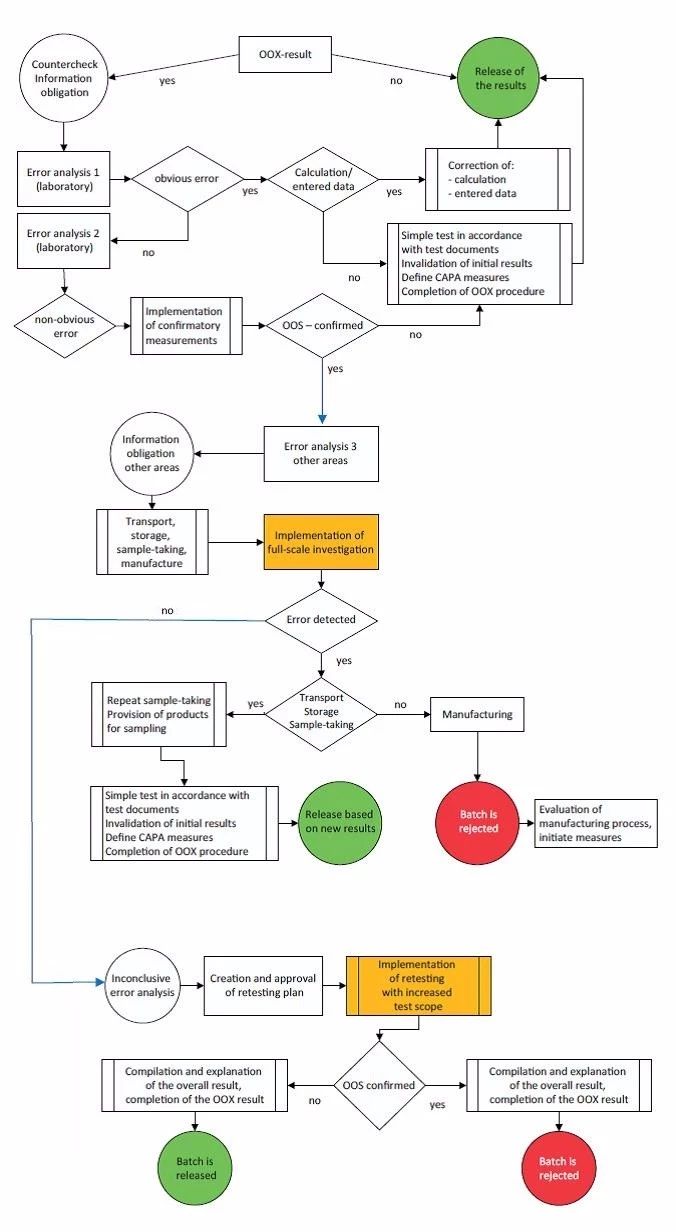
OOS调查流程:
文章来源:GMP办公室
本网站刊载的所有内容,包括文字、图片、音频、视频、软件等,如非标注为“原创”,则相关版权归原作者所有,如原作者不愿意在本网站刊登相关内容,请及时通知本站,我们将第一时间予以删除。