扫一扫 添加小助手
服务热线
13818320332
扫一扫 关注我们
 1月18日,欧洲药典(EP)发布公告,针对EP增补版10.5的实施对于CEP持有人的影响,进行了相关沟通:
1月18日,欧洲药典(EP)发布公告,针对EP增补版10.5的实施对于CEP持有人的影响,进行了相关沟通:

图:EP增补版10.5相关公告
Supplement 10.5 of the European Pharmacopoeia (Ph. Eur) is now available. CEP holders are invited to update their applications according to the revised monographs that will be implemented on 1 July 2021, and to follow the instructions given below.
EP增补版10.5现已发布,将于2021年7月1日实施。请CEP持有人根据该版本专论更新其申请,并遵循以下指示。
The table at the end of this announcement provides a list of substances covered by a Certificate of Suitability to the monographs of the European Pharmacopoeia (CEP) and for which a revised monograph will be implemented on 1 July 2021 in Supplement 10.5 of the Ph. Eur.
本公告列出了CEP证书所涵盖的相关API,这些API专论已修订,并将于2021年7月1日在EP增补版10.5中实施。
According to Directives 2001/83/EC and 2001/82/EC, as amended, it is the responsibility of the manufacturer to comply with the current version of a Ph. Eur. monograph, and therefore to update the specification when a revised monograph is issued. In addition, the European Directorate for the Quality of Medicines and HealthCare (EDQM) ensures that CEPs refer to the most recent version of a Ph. Eur. monograph at any time.
根据修订的法令2001/83 / EC和2001/82 / EC,生产商有责任遵守当前版本的EP专论,因此在发布修订的专论时要更新质量标准。此外,欧洲药品和健康质量管理局(EDQM)确保CEP引用EP专论的最新版本。
The need to submit information to the EDQM following a revised monograph depends on the changes made to the monograph. Updates to the monographs are classified by the EDQM into two categories, labelled “Case A” and “Case B”, and this influences the information required. In the list of revised monographs below, it is indicated which classification (“Case A” or “Case B”) is applicable. In addition to this web announcement, the EDQM, as a courtesy, will contact CEP holders with details of how to proceed for the dossiers impacted by the revised monograph(s). However, it remains the responsibility of the CEP holder to comply with the requirements of the monograph and if necessary to update their respective applications by the implementation date of the revised monograph at the latest, regardless of whether they have been contacted by the EDQM.
考虑到专论修订后是否需要向EDQM提交信息,这取决于对专论所做的变更。EDQM将专论的更新分为两类,分别标记为“情况A”和“情况B”,这会影响所需的信息。在下面的修订的专论列表中,指出了适用的分类(“情况A”或“情况B”)。除此网络公告外,EDQM还将与CEP持有人联系,详细说明如何处理受修订专论影响的卷宗。但是,CEP持有人有责任遵守专论的要求,并在必要时,最迟在修订的专论的实施日期之前,更新其各自的申请,无论EDQM是否已联系他们。
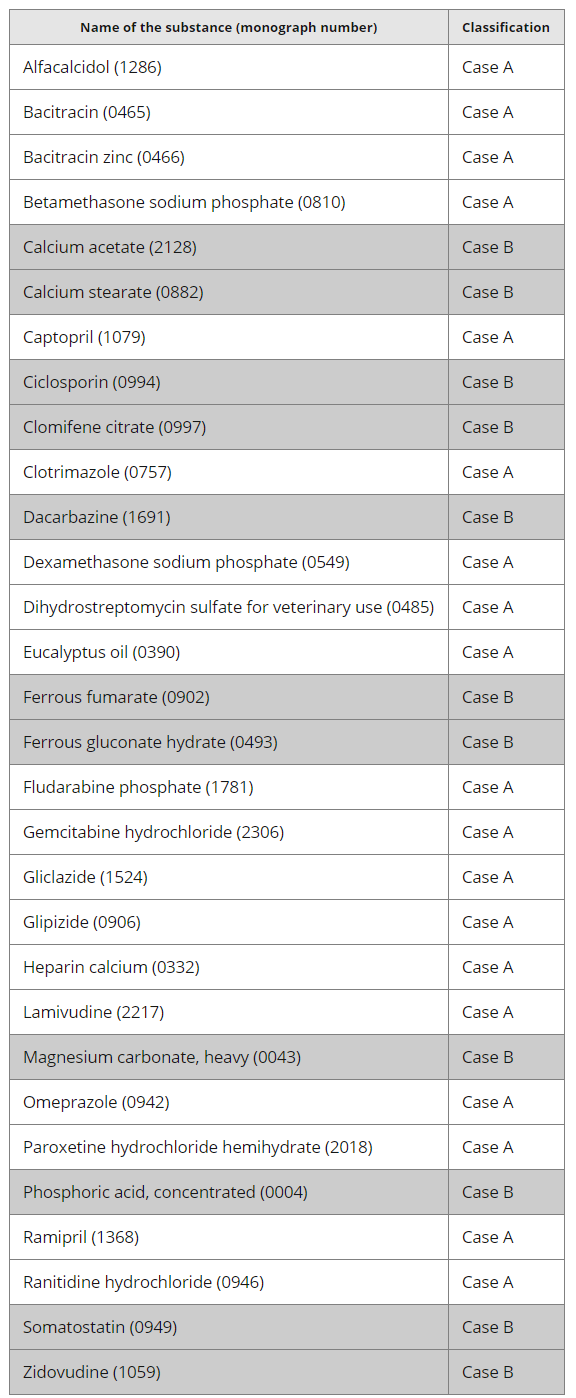
图:情况A和情况B涉及到的API
情况A Case A
The specification of the substance should be updated according to the revised monograph. Unless the CEP holder has made reference to the “current version of the monograph” (without providing details on the Ph. Eur. tests and methods in the CEP application), the updated specification should be included in the next request for revision that is submitted to the EDQM (minor, major or renewal of the certificate) and identified as such at that time (such an update will be free of charge). Where the CEP holder has made reference to the “current version of the monograph”, the revised monograph should be implemented without the need to update the specification of the substance at the next request for revision.
应根据经修订的专论,对该API的质量标准进行更新。除非CEP持有人已提及“专论的当前版本”(未在CEP申请中,提供有关EP检验和方法的详细信息),否则,应提交更新的质量标准,这需要包含在下一个给EDQM的修订申请中,(证书的次要、主要变更或更新),届时需要进行说明(此类更新是免费的)。如果CEP持有人提及“专论的当前版本”,则应执行修订后的专论,而无需等到在下次修订申请时,再更新该API的质量标准。
情况B Case B
This case concerns amendments to the monograph which require the submission of data to the EDQM.
此情况涉及到的专论修订,要求向EDQM提交数据。
An updated dossier demonstrating that the substance complies with the requirements of the revised monograph should be provided within three months of the EDQM contacting the CEP holder. The company is asked to provide a Module 1 briefly discussing the changes made to the application. This module should also include a clarification on whether all related substances are controlled using only the method described in the revised monograph and whether the substance contains any impurities which are not described in the revised monograph (and which are found above the reporting threshold of the Ph. Eur. general monograph 2034).
在EDQM与CEP持有人联系后的三个月内,应提供一份更新的卷宗,以证明该API符合修订后的专论的要求。要求公司提供模块1,简要讨论对申请所做的变更。该模块还应澄清以下内容:是否仅使用修订后的专论中描述的方法来控制所有的有关物质,以及该API是否包含修订后的专论中未描述的任何杂质(并且发现的含量超出了EP一般专论2034的报告阈值)。
Module 3 should be updated to include, as necessary:
如有必要,模块3应进行更新,以包括:
a comparison of the impurity profile of the substance with the updated transparency list of the monograph (3.2.S.3.2 “Impurities”, 3.2.S.4.5 “Justification of Specifications”);
a discussion on the suitability of the revised monograph to control any impurities which are not described in it;
an updated substance specification/test methods description (3.2.S.4.1 “Specifications”, 3.2.S.4.2 “Analytical Procedures”);
certificates of analysis of two batches with reference to the revised monograph (3.2.S.4.4 “Batch Analysis”);
validation and cross-validation data when an in-house method is used as an alternative to a new test method in the monograph (3.2.S.4.3 “Validation of Analytical Procedures”).
将API的杂质分布与专论的更新列表进行比较(3.2.S.3.2“杂质”,3.2.S.4.5“质量标准合理性”);
讨论修订后的专论是否适用于控制其中未描述的任何杂质;
更新的API质量标准/检验方法说明(3.2.S.4.1“质量标准”,3.2.S.4.2“分析程序”);
参照修订专论对两批次进行分析的证书(3.2.S.4.4“批次分析”);
当使用内部方法代替专论中的新检验方法时,验证和交叉验证数据(3.2.S.4.3“分析程序的验证”)。
In the “Case B” scenario, CEP applications require an update and therefore any holder of a CEP for a substance in the “Case B” list below is expected to provide the requested information to the EDQM, even if no specific request for information was received (this may happen namely when information regarding a change of contact person has not been submitted to the EDQM in a timely manner).
在“情况B”场景中,CEP申请需要更新,因此,即使没有明确的信息提交要求,对于以下“情况B”列表中API的CEP持有人,都应向EDQM提供所要求的信息。(EDQM没有发出信息提交要求,这可能是在未及时将有关变更联系人的信息提交给EDQM时发生的)。
If the requested information has already been presented in the approved dossier, a simple letter stating this is deemed sufficient.
如果所要求的信息已经存在于批准的档案中,则只需写一封简单的信,说明已足够。
Failure to update the CEP application and to provide data to the EDQM may challenge the validity of the concerned granted CEP, or delay the ongoing evaluation process of the concerned application.
未能更新CEP申请或无法向EDQM提供数据,可能会挑战有关授予的CEP的有效性,或延迟有关申请正在进行的评估过程。
Upon receipt, data will be reviewed within 3 months and the CEP holder will be informed of the outcome of the evaluation. The assessment may also result in a revised CEP being granted.
收到信息后,EDQM将在3个月内审查数据,并告知CEP持有人评估结果。评估还可能导致授予修订后的CEP。
This procedure is free of charge, unless the holder submits, at the same time, a request for other changes.
除非持有人同时提出其他变更请求,否则此程序是免费的。
Ref.: Implementation of the European Pharmacopoeia Supplement 10.5 – Notification for CEP holders. 18 JANUARY 2021. EDQM.
文章来源:PharmLink
本网站刊载的所有内容,包括文字、图片、音频、视频、软件等,如非标注为“原创”,则相关版权归原作者所有,如原作者不愿意在本网站刊登相关内容,请及时通知本站,我们将第一时间予以删除。